Clinical Trials >> Chronic Heart Failure (Starting October 2024)
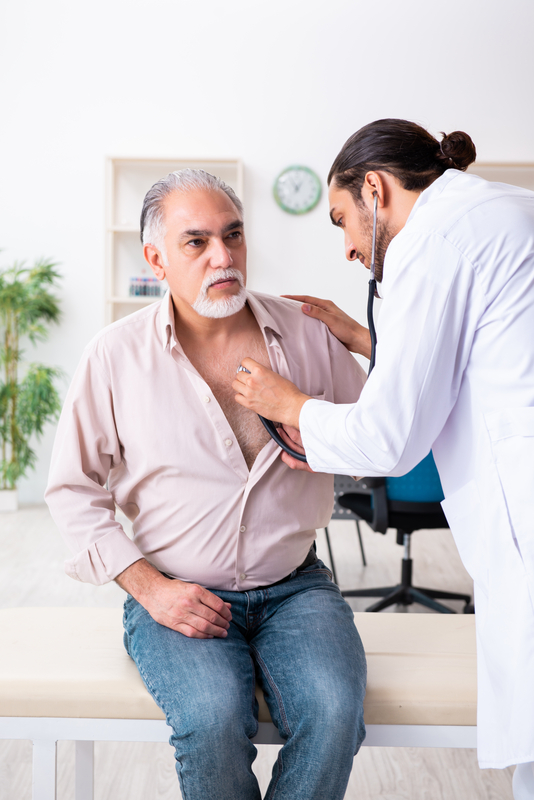
Chronic Heart Failure (Starting October 2024)
Despite recent advances in the management of Heart Failure (HF), an unmet need for effective therapies remains, especially for HF with LVEF ≥40%. Established therapies for heart failure with mildly reduced and preserved ejection fraction include the management of risk factors and comorbidities, addressing weight loss for participants with obesity, as well as the control of signs and symptoms, including diuretics for fluid retention.
The purpose of this clinical research trial is to look at the effectiveness, safety, and tolerance of combining the investigational trial drug with empagliflozin in participants diagnosed with heart failure (HF). Empagliflozin is approved by the FDA for the treatment of heart failure.
The study will last approximately 1.5 years up to 3.5 years with 18 clinic visits.
A few qualifications to participate are:
- You have been diagnosed with Chronic HF for at least 3 months and have an ejection fraction of at least 40%
- Currently receiving treatment for your HF
Qualified participants will:
- See a physician at no cost
- Receive pertinent tests and imaging at no cost
- Receive compensation for time and travel




































































 - web design by teaminhouse
- web design by teaminhouse